Services
Claims assessment
Regulations make finished product manufacturers to constantly looking for ingredients with clinically substantiated claims that are truthful and not misleading. MHRI can assist dietary ingredient manufacturers in making clinically substantiated claims in line with the local regulatory requirements.
Structure/function claims for dietary supplement ingredients in the US
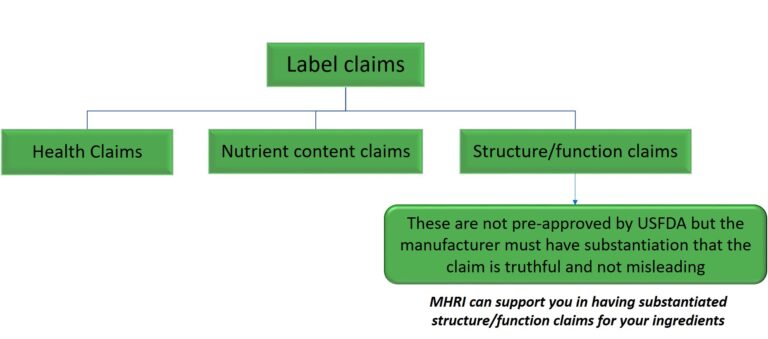
In the US, label claims are used for conventional foods and dietary supplements. USFDA defines three categories of label claims viz., health claims, nutrient content claims and structure/function claims. Structure/function claims describe the role of a nutrient or dietary ingredient that is intended to affect the normal structure or function of the human body, for example, “calcium builds strong bones”.
Structure/function claims are not pre-approved by FDA, but the manufacturer must have substantiation that the claim is truthful and not misleading. If a dietary supplement label includes such a claim, it must state in a “disclaimer” that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not intended to “diagnose, treat, cure or prevent any disease”.
Use Dietary Supplement Claim Checker to quickly verify your structure/function claims. MHRI can suggest structure/function claims for your ingredients based on clinical evidence.
Permitted indications for Listed Complementary Medicines in Australia
Herbal products are regulated as Complimentary Medicines under the Therapeutic Goods Act 1989 by the Therapeutic Goods Administration (TGA). Complementary medicines may be either listed or registered, depending on their ingredients and the claims (permissible indications) made. Listed complementary medicines can only contain certain low risk ingredients in acceptable amounts that are permitted for use in listed medicines by the TGA. They must be manufactured in accordance with the principles of Good Manufacturing Practice (GMP) and can only make indications (for therapeutic use) for health maintenance and health enhancement or certain indications for non-serious, self-limiting conditions.
The therapeutic indications on listed medicines are not evaluated by the TGA at the time a medicine is listed on the ARTG however, manufacturers of Listed complementary medicines in Australia often interested in using herbal ingredients with differentiated “Permissible indications” (claims). This is because, they must certify that they hold the evidence to support indications and claims made for their medicine and that this information be made available to the TGA upon request.
MHRI can evaluate the clinical evidence available on your herbal ingredients and suggest suitable permissible indications for your ingredients.
GRAS consultations for the US market
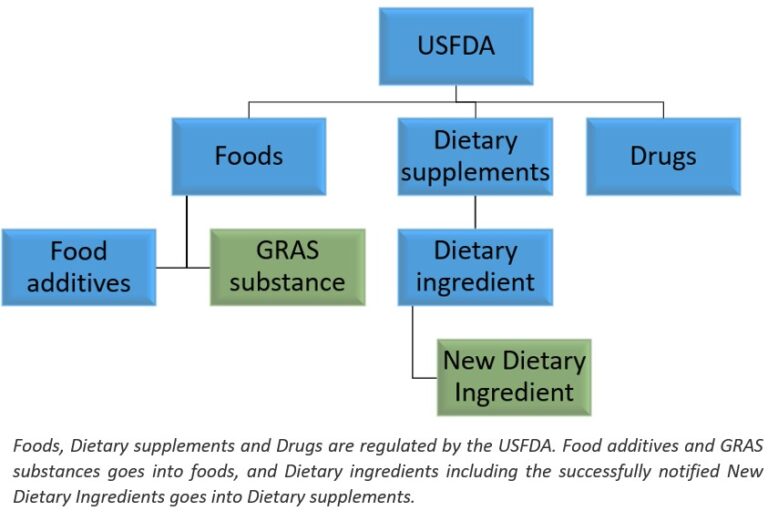
GRAS is the shortened form for “Generally Recognized as Safe”. In the US, if a substance is to be added into foods, then the substance should either be a food additive or a GRAS substance. Food additive has to undergo premarket review and approval by the US FDA before it gets added into foods. GRAS substances on the other hand can be added into foods after voluntarily notifying to the US FDA or after having achieved Independent GRAS conclusion (called as Self-GRAS). It is to be noted that same standards apply to a conclusion of GRAS status regardless of whether the GRAS status conclusion is notified to the US FDA or not.
MHRI can assist you getting GRAS status for your ingredient. GRAS can be self-GRAS or FDA notified GRAS.
NDI consultations for the US market
New Dietary Ingredient (NDI) is a dietary ingredient that was not marketed in the United States in a dietary supplement before October 15, 1994. The Federal Food, Drug, and Cosmetic Act (the FD&C Act) requires that manufacturers and distributors who wish to market dietary supplements that contain “new dietary ingredients” notify the Food and Drug Administration about these ingredients. The notification must include information that is the basis on which the manufacturer or distributor has concluded that a dietary supplement containing a new dietary ingredient will reasonably be expected to be safe under the conditions of use.
MHRI can assist you in determining if your ingredient is an NDI and can assist in assessing the safety of NDI in dietary supplements under the recommended conditions of use.
Novel Food consultations for the EU market
Novel Food is defined as food that had not been consumed to a significant degree by humans in the EU before 15 May 1997. If your food or food ingredient is considered a novel food, then it must be authorized before placing on the EU market. There are ingredients that are novel for use in foods but are “not novel” for use in food supplements.
Listen to this concise, podcast-style overview of the European Union’s Novel Food Regulation (EU) 2015/2283 and the implementing Regulation (EU) 2017/2469, covering key requirements and scientific data expectations.
MHRI can assist you in assessing if your ingredient is novel or non-novel when used in food or food supplements.
Natural Health Product License from Health Canada
In Canada, herbal remedies, homeopathic medicines and traditional medicines such as traditional Chinese and Ayurvedic medicines are regulated as Natural Health Products by Health Canada under Natural Health Products Regulation. More than 70% of Canadians have used natural health products like vitamins and minerals, herbal products, and homeopathic medicines. As per the Natural Health Products Regulations, product and site licenses are required before a natural health product can be marketed in Canada.
MHRI can assist you in getting site license for your facility and product license.
Project management
Whether it is a new product development or improvement of existing product or implementation of new process that includes training to employees, the objectives have to be met within a specified time period and within the allocated budget. Objectives of your new initiative can be better achieved through effective project management without compromising existing business.
MHRI can effectively manage your new projects with prompt status update to the management through its completion.
Clinical study protocol writing services
A clinically study protocol is a critical document that serves as a foundation for conducting any clinical study. It helps to ensure the clinical studies are conducted in compliance with Good Clinical Practice. A clear and well written protocol helps everyone involved in a clinical study to be on the same page before initiating the study and makes the approval from Institutional Review Board (IRB) quicker and easier. A well written helps reduce the need for protocol rewrites and updates.
MHRI can provide you a clearly written clinical study protocol.
Scientific writing services
MHRI can assist you in writing a research project proposal for grant or publication of a scientific study in a journal or converting your scientific study reports in to a product promotional material.
